The Anti-Mouse
Could a hairless African rodent be our secret weapon in the war on cancer?
In the outskirts of San Antonio, Texas, where guys dressed like the Marlboro Man loaf at gas stations and kids hang out at two-step dance halls, a sarcastic 55-year-old biologist from Zimbabwe is getting frustrated. She's only trying to do what thousands of her research colleagues do every week of the year. She's trying to make an animal sick. She wants to give cancer to a rodent.
There's a problem with the colonies of naked mole rats that Rochelle Buffenstein carted down to her new lab in Texas four years ago. Unlike mice, which die of cancer by the crateful, not a single one of her animals has ever developed a naturally occurring tumor. Nor has any other naked mole rat seen anywhere in the world.
If you want to know how cancer grows and how it kills, then it helps to have a case in front of you. Most researchers don't wait for tumors to pop up by chance: They irradiate their animals, or transplant tumors from one to another, or inject carcinogens directly into their bellies. In 2004, Buffenstein and her students tried one of these shortcuts. They placed some mole rats in a gamma chamber and blasted their pale, pink bodies with ionizing rays. The animals were unimpressed. When I visited Buffenstein’s lab this past July, many were still alive, skittering through the plastic tubes of their basement habitat at the Barshop Institute for Longevity and Aging Studies.
Four years later, Buffenstein tried again. This time she infected cells from a naked mole rat with a virus designed to corrupt their nuclei with the cancer-causing genes SV40 TAg and Ras. Then she slipped those cells into a live mouse, under the skin behind its ear. If you do the same using infected material from a mouse or a rat, or even a cow or a human, the transplant quickly grows into a deadly tumor, invading nearby fat and muscle tissue. But when Buffenstein and her colleagues used cells from a naked mole-rat, nothing happened.
Photograph by Jessie Kotler.
That's how science goes: A hundred failures for every success. Earlier this year, one of Buffenstein's graduate students tried smearing the skin of half a dozen naked mole rats with a pair of vicious carcinogens: A synthetic compound called DMBA and an inflammatory agent known as TPA. When the same toxic pairing was applied to regular Black-6 lab mice as an experimental control, a cluster of tumors popped up within weeks. Every single mouse had cancer, and every single mouse died. The naked mole rats went on skittering through their tubes.
Her latest assault involves pouring carcinogens down the mole rats' throats in a last-ditch effort to induce liver or mammary cancer. But that may not work, either. For years, Buffenstein's laboratory Rasputins have been irradiated, poisoned, and heated up; their cells dosed with every imaginable pollutant—chemotherapies, oxidative stressors, and heavy metals—with little or no effect. "You name it," the professor says, "we tried all the kinds of toxins that are out there, and the naked mole rat seems to be very resilient and resistant." A drug regimen that would be murderous to mouse cells must be doubled or tripled or even multiplied 50 times over, she explains, before it would have similar effects on a naked mole rat.
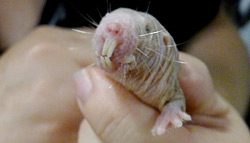
When Buffenstein takes me downstairs to see the animal colonies, we're not wearing gloves or gowns. At the massive rodent-breeding facilities in Massachusetts and Maine, where standard lab mice are manufactured by the millions, industrial technicians stalk around in what look like spacesuits, and the animals are housed in airtight isolator cages or ventilated barrier rooms that protect them from a list of 40 specific pathogens. Here in Texas, I've got a precious mole rat squirming, unprotected, in the palm of my hand. Its forebears were exported from a maze of burrows under a sweet-potato field in equatorial East Africa, but Buffenstein's not worried about my germs. I couldn't infect this animal if I tried.

Photograph by Jessie Kotler.
The naked, 5-inch thing between my fingers, slightly bulbous at the hindquarters and topped with a set of lurid buck teeth, resembles nothing so much as a penis dentatus. Its pink skin, which feels as delicate as a moistened tissue, belies an astounding stamina. Mole rats have spent the last 24 million years adapting to a life spent in dark and humid tunnels six feet underground. Free of the terrestrial predators and frost that terrorize field mice, they've been left to wander down their own labyrinthine evolutionary path: In place of a mouse's fur, they have tiny hairs that brush against the walls to help them navigate; in place of a mouse's ears, they have a pair of pinprick holes on the sides of their heads; in place of a mouse's eyes, they have meager, malformed slits; and in place of a mouse's fragile constitution, the naked mole rat has what could be the most extraordinary set of natural defenses ever found in a mammal. A mouse's life is short and terrible—even in the lab, with plenty of food and a steady thermostat, it lasts for just three or four years at the most. A naked mole rat shows no sign of aging until it's a quarter of a century old. Blind and plump, it skitters around in a hazmat suit of its own creation.
That's why Buffenstein has gone to such lengths to nurture and sustain her exotic colonies, when she might just as well have filled her cages with bulk-rate mice from Charles River. Most biomedical researchers want to make animals sick so they can try to find a cure. Buffenstein does things the other way around. Instead of using rodents as a model of human disease, she takes them as a model of human health. Naked mole rats never get cancer; they cure themselves. She's determined to figure out how they do it.

Photograph by Jessie Kotler.
In a paper co-authored for an animal research journal earlier this year, Buffenstein laid out the case for using the naked mole rat in the study of age-related chronic disease. With some work, she wrote, this animal could be something more than a quirky alternative to the mouse: a standard model on its own terms—a "supermodel," even, for certain fields of medicine. If you're interested in the progression of cancer or the biological mechanisms that promote its growth, she says, then a factory-made lab mouse might be your best option. "But as soon as you're looking at how to abrogate a process"—as soon as you start asking how we might go about preventing cancer in the first place, or slowing the decline of our bodies—"then I don't think [mice] are the right model at all."
It's conventional wisdom among scientists that the path to insight lies along a vertical plane, a descent into the details. "We are so ingrained to think that the best discoveries will come by studying in depth one species and not seeing the animal in a holistic perspective," says Buffenstein. Indeed, no mammal on Earth has been plumbed to such profundities as the inbred lab mouse. At some point, though, we may discover that we've been drilling in the wrong place—that other animals have secrets, too. Could the naked mole rat be hiding the most coveted one of all?
* * *
Among all the causes of human death, natural or man-made, cancer stands at No. 2. Nearly 570,000 Americans succumb to the disease each year—about one of every four deaths. That's despite a staggering outlay of research funding: More than $5 billion annually, and hundreds of billions spent in total since the passage of the National Cancer Act in 1971. Per capita, the United States government puts seven times more toward oncology research than the European Union, yet our practical gains have been modest at best, with mortality rates falling for some types of cancer (breast, colon) while rising for others (skin, liver). The War on Cancer looks like a quagmire.

Photograph from the National Library of Medicine.
The modern study of the disease, and search for a cure, has from its outset relied on armies of inbred lab mice, mobilized for the long engagement by policymakers in Washington.In 1938, the year after the founding of the National Cancer Institute, the feds designated Clarence Little's mouse factory in Bar Harbor, Maine as a central training ground for research animals.* By the end of the 1940s, Little's Jackson Laboratory had more than doubled its mouse production, and soon hordes of cloned rodents were being marched to the front lines, injected or implanted with tumors, then bombarded with whatever chemical agents might shrink their tiny cancers. In 1974, a massive government research program was sifting through 3 million mice and more than 40,000 compounds every year. These same animals, purchased by the pallet from private breeders, dominate the field now more than ever: About three-quarters of all the recent animal cancer studies in the PubMed database draw their data from the mouse.
From the start, rats and mice had a distinct advantage over other model systems: They're small and cheap; they reproduce readily in the lab; their genes and lineage are easy to track. But the mouse, in particular, appealed to cancer researchers on account of its exquisite sensitivity to the disease. If a naked mole rat can't seem to get cancer on its own, a mouse can't seem to avoid it. Look too hard at a commercial Black-6, and it grows a lump: Seven out of 10 develop naturally occurring tumors. Other inbred mice are even more vulnerable, with spontaneous cancer striking more than 99 percent in some strains. (Wild mice, too, show a predisposition to cancer.) When Clarence Little hawked his factory-made organisms to the honchos at NCI in the 1930s, he was offering access to something more, or something less, than a mere laboratory animal. He'd created a line of cheap, disposable, tumor-making machines.
Consider that a man is 3,000 times bigger than a mouse; that he lives 40 times longer; that, as a result, his cells reproduce 100,000 times more often before he dies. To stave off malignancies, a human body must safely negotiate quintillions of chromosomal splits in a lifetime, versus mere trillions for a mouse. Yet a raft of genetic battlements prevent our nuclei from going haywire. Human cells, for example, have a built-in self-destruct mechanism that mouse cells lack—a molecular scissors that lops off bits of DNA with each go-round of the cell cycle. Cultured in a dish, human fibroblasts reach a natural endpoint—a state called "replicative senescence"—after around 50 cell divisions. For a would-be cancer to circumvent our natural defenses, and send a human cell multiplying out of control, it takes no fewer than six genetic mutations—six random, unlucky breaks. The same can happen to a line of mouse cells in just two steps.

Photograph from Charles River Laboratory.
What makes life harder for the mouse helps the scientist. By 1980, we knew of at least 1,600 chemicals that caused cancer in rodents, compared to just 15 for humans. Researchers could manufacture a cancer of the plasma cells by injecting a mouse's belly with mineral oil, or a colon cancer by feeding it aromatic hydrocarbons, or any of a panoply of other tumor types simply by infusing the right carcinogen into a mouse's bloodstream. An endless line of experimental subjects, cancer patients by proxy, could be made with chemical ingredients, or through other more direct means: Early drug-screening efforts implanted mice with leukemia or breast-cancer cells that had been sucked from the tumors of other mice; modern studies make use of the xenograft model, in which bits of human cancer tissue are placed into mice with poorly functioning immune systems. The most common vehicle for the latter—a flimsy, inbred strain that lacks a viable thymus—happens to be as pink and hairless as a naked mole rat. So much the better for watching tumors grow.
Nearly all of the cancer drugs we have today—some useful, others miraculous—came about as a result of this method. Modern chemotherapy regimens were devised according to a kludgy logic: Ply your rodent models with as many different chemicals as you can, then combine the best to find a drug cocktail that works (never mind why or how). A complementary approach, now in favor, takes more forethought: Researchers use transgenic mice and other tools to figure out exactly which of a cancer's genes and molecules make it thrive, and then try new drugs custom made to disrupt them. Whether you're using brute force or more targeted attacks, though, the path to the clinic runs through the mouse: Cages and cages of them, black ones and brown ones, albinos and nudes. It's a system designed for efficiency, designed to churn out tumors at maximum speed. But the industrial model of cancer research may only be slowing us down.
What's wrong with the lab mouse? The cancer it gets with such preternatural ease bears only a modest relation to our own. Mice are prone to lymphomas and sarcomas, the sorts of tumors that might crop up in blood or bones or cartilage. Humans tend to get carcinomas—cancers of the breast, the prostate, the lung, and the skin. While it’s true that certain rodent strains, like Black-6, have been bred to develop mammary lumps or other more humanlike diseases, these resemble rare, hereditary syndromes—an implacable family history of cancer, perhaps—rather than the sporadic cases that affect most people. Spontaneous cancers of the colon, bladder, stomach, or bronchi rank among the most common in humans but are virtually unknown in mice or rats.
Transgenic models may be limited in other ways. The molecular tweaks that can induce a tumor in the lab typically involve the wholesale deletion of certain genes, or their gross overexpression. Human cancers, by contrast, tend to result from more specific factors—one-for-one switches of nucleotides that affect some cells but not others, or alter a gene's function in subtle ways. Transplanting real, human tumors onto the bodies of mice gets around a few of these problems but introduces new ones. A xenograft rarely spreads within a mouse, so it can't really be used to study one of the most deadly aspects of human disease, metastasis. Nor does it provide an accurate picture of how a tumor might interact with healthy human tissue or coexist with the forest of bacteria that live inside our bodies.
All this adds up to a big problem: Mouse cancer as it’s studied in the lab has become as standardized as the animals themselves. In place of the organic and varied tumors that turn up in the hospital—a menagerie of cancers that evolve on separate paths within each patient—industrial science works with a bland and homogenized product, a fast-food version of the disease. A few years ago, Robert Weinberg, the MIT biologist who in 1982 discovered the first clear link between tumor-causing mutations in rodents and humans, told Newsweek's Sharon Begley that lousy animal models have become "the rate-limiting step in cancer research." It wasn't the first time he'd come out against xenografts and other rodent tools. In another interview, with Clifton Leaf of Fortune, Weinberg said that drug companies were wasting hundreds of millions of dollars on animal research that has little predictive value.
It's an old joke that scientists have cured mice of cancer a hundred times over, while humans continue to suffer. According to Derek Lowe, an acerbic chemist who works for the pharmaceutical industry and blogs at In the Pipeline, lab-made rodent tumors are well-known to be "horrible, just horrible" stand-ins for human disease. As with tuberculosis, for every cancer cure that makes its way from mouse to man, many more stop short of the clinic at great expense. No one in R&D trusts the animal models, writes Lowe, "as anything more than rough, crude filters on the way to clinical trials."
Few scientists would deny these flaws in the standard cancer models—yet fewer still would argue for an alternative. The mouse is the worst kind of lab animal, they say, except for all the others that have been tried. No organism does a better job of generating cancers; no animal provides more research value per dollar spent; no tool serves up more data for publication. What gaps remain between the mouse and human forms of cancer might one day be bridged with genetic engineering—soon we'll be running tests in "humanized" animals, embedded with bits of our own DNA. That's the dream, at least: Keep the strategy but refine the tactics. Having come this far, retreat would be unthinkable. So science will do what it always does: Go deeper, deeper, deeper.
* * *
Down in the basement, the mole rats are quiet; nothing is stirring. They cower in plastic boxes lined with shredded paper and linked up with 2-inch tubes. One timorous beastie finally noses its way out of the pile, and wriggles down a passageway toward a neighboring box. Until the 1890s, no specimen of these shy animals had ever been seen in the West, and they weren't studied in captivity until the 1960s. Even today, the husbandry of naked mole rats is more an art than an industry.
Everyone who cares for Heterocephalus glaber has her special formula; Rochelle Buffenstein's happens to taste like cream of wheat. She pours a few tablespoons of yellow powder into a bowl and stirs in some water to make a paste. Lab mice eat hard food pellets made from processed grains, minerals, and ground-up pork products. Naked mole rats—at least the ones in Buffenstein's lab—get "Pronutro," a South African breakfast cereal of corn, soy, and chicory. "It's designed for sportsmen," she says, proffering a spoon. "Maybe that's why the animals live so long."
Another theory: It's the chunks of fresh produce she leaves in their bedding every afternoon. At Charles River and the Jackson Lab, the mice nibble their pellets at any time of day. The mole rats in Texas enjoy a wholesome lunch: corn and green beans, yams, some fresh garlic or a chunk of banana. As we discuss their menu, I think of the factory mice in shoebox cages and their all-you-can-eat buffets. Mark Mattson, a senior scientist at the National Institute on Aging, calls the standard lab rodents "couch potatoes" and argues that feedlot rearing makes them unfit for medical research. The basement at the Barshop looks like an animal health spa by comparison: Between meals of fresh fruits and vegetables, the naked mole rats are free to scamper through a plastic maze and tussle with their siblings.
The stillness from before has by now subsided. The colonies are more comfortable with the humans in the room, and they resume their busy work: Inscrutable forays from one box to another, scored with high-pitched chirps. A mouse facility tends to silence (but for the sound of light rock piped in through speakers). Naked mole rats, once they've settled down, can deliver 19 distinct vocalizations within the human range of hearing.
The longer we stand there, the louder the chirping becomes, until another sound starts to take its place—a faint clicking noise that builds to a clatter. The mole rats have taken up the activity for which they're most ostentatiously designed: They're biting at the corners of the habitat with their momentous teeth. If one's not careful, the animals can chew their way out, says Buffenstein. (I've read elsewhere they can bite through concrete.) By this point it's getting hard to continue the interview. We have to raise our voices above the clack-clack-clack.

Photograph by Jessie Kotler.
We step into another animal room, where Buffenstein keeps the naked mole rats' big, hairy relatives from Namibia. One of these—a Damaraland mole rat—slithers into a tube when we arrive and begins stomping its hind-paws on the floor, brushing them backwards. It's an alarm signal for the hive, and mole-rat siblings soon pick up the call, turning their bodies around and brushing their own feet in turn. This lighting of the beacons proceeds throughout the tubes for several minutes, until a scruffy platoon converges to defend the chamber beside my elbow.
Both naked mole rats and their Damaraland cousins live as ants or termites do, in a rigid, insectile society. A lone female produces all the offspring, while lesser castes forage, defend the colony, and care for her young. The naked mole rat queen, bigger and longer than her peers, stomps around and bullies her workers to keep them in line. In the other room, I saw one barge into a plastic box and muscle a scrawny male into what looked like a rodent 69. She was readying him to breed, Buffenstein told me; a queen usually takes between one and three consorts. I watched them squirm together, head-to-tail. It was obscene.
No other mammals are known to assemble like this, under a monarch's rule. Naked mole rats are even more bizarre for their smooth, pink skin (mammals have fur) and their enfeebled eyes (other mole rats see just fine). Like reptiles, they have trouble controlling their temperature (also strange; rodents are warm-blooded). As a matter of taxonomy, the naked mole rat is closer to a guinea pig or porcupine than a mouse or a rat, but really it's neither one nor the other. Buffenstein knows that she's working with an oddball; she did a lot of the work that proves it. "[The naked mole rat] does have very unique mechanisms that are not seen in other animals," she says, referring both to its superficial quirks and to whatever private biochemistry helps it to shrug off cancer, deflect toxic chemicals, ignore painful stimuli, and otherwise live five times longer than one might expect.
It's rare to hear a biologist indulge in such special pleading. The logic of model organisms depends, first of all, upon their essential blandness: If one life form is going to stand in for all the others, like a canvas that can be painted with disease, then crumpled up and thrown away, it ought to be bleached of unnecessary details. The perfect lab animal—the lumper's tool—would be uncontaminated by evolutionary oddities and stripped of genetic clutter: A dog that's not too doggy, say, or a rodent that's bred to be just average. That's another reason why scientists love to work with mice: They're boring.

Photograph by Jessie Kotler.
Buffenstein pulls a Damaraland mole rat out of its cage so we can give it a closer look. There were mole rats on the farm in Zimbabwe where she grew up—"pests, as my father would call them"—but she didn't give them much thought until, as an undergraduate, she started collecting them in Kenya for research. Her post-doctoral work on bats, lizards, and kangaroos took her to Australia; at one point she rescued a baby 'roo from a fur trapper and carried it around for a while in her handbag. Even now, as a tenured professor at the University of Texas, Buffenstein's projects still have a bit of that fuzzy-edged, put-a-kangaroo-in-your-pocket feel. She tells war stories about her fellow mole-rat obsessives—people who schlep around the country, trading colonies for zoos, labs, and private homes. After picking up a batch in Arizona, her flight home was canceled, so she released the mole rats into the bathtub of an airport motel. The next morning, the cages were tucked under her legs like carry-on luggage and covered over with the financial section of the New York Times.
Aging and cancer research are hardly at the fringe of biomedicine, but Buffenstein's is a science of the weird. Where most of her peers get their animals prepackaged from industrial facilities, she takes hers straight off the farm—plucked from the soil, even—like heritage turkeys or heirloom potatoes. "I think [the use of mice] is constraining us to answer questions for which the mice are best-suited," she says. Her own laboratory rodent, as gnarly as a farm-stand fruit, offers an alternative. It's the anti-mouse.
* * *
Ten years ago, Buffenstein was one of just a handful of biologists studying naked mole rats in captivity; now her field comprises some three dozen labs around the world. Her colleagues have looked at why naked mole rats are immune to the pain caused by spicy foods, or how they avoid getting itchy when doused with histamine, or what allows their brains to get by without much oxygen and a shriveled pineal gland. In Rochester, N.Y., a pair of Russian-born biologists, Andrei Seluanov and Vera Gorbunova, are devoted to finding out exactly how naked mole rats keep from getting cancer. A couple of years ago, they announced they'd found a "clue."
The discovery grew from previous work, for which the husband-and-wife team had gathered tissue samples from a caboodle of rodent species, minced them up, and watched them grow in culture. (Some of the animals came from laboratory suppliers; others from local hunters, who shipped the carcasses to Rochester in Styrofoam boxes.) Recall that a normal human cell will split some 50 times before spinning down, while a mouse cell tends to divide with abandon. Seluanov and Gorbunova found that among their bigger rodents—the beaver, the porcupine, and the capybara—fibroblast cells behave like those from humans, reaching a senescent state that serves as a natural bulwark against cancer. But no such effect could be found in the naked mole rat, they claimed, nor in its similarly long-lived cousins, the grey squirrel, the chinchilla, and the chipmunk. These animals must have evolved some "alternative mechanisms" for keeping tumors at bay.

Photograph by Jessie Kotler.
The Russians' follow-up paper, published the following year, zeroed in on just two species—the naked mole rat and the standard Black-6 mouse. The scientists scraped fibroblasts from the lungs and underarm skin of each and plated them in the lab. When they checked back a week later, the dishes of mouse cells had filled out into a dense layer of growth while the naked mole rat cells remained loosely packed. It's normal for cells to stop dividing once they're close together—in a process called "contact inhibition"—but the naked mole rat samples appeared to stagnate even when they were just barely touching. To Seluanov and Gorbunova, this suggested the presence of a novel backstop against cancer, one that had never been seen before in any species. A naked mole rat cell, they argued, is hypersensitive to crowds: The minute one of its cytoplasmic arms brushes up against a stranger, it stops dividing. A cell could be induced to overcome its shyness with the help of some cancer-causing proteins, and so emboldened it would grow out of control, like a tumor. But even then, a second line of defense would be activated: The cell would apoptose, or self-destruct.
If these findings hold up, it's not clear how they might be marshaled to improve human health, since the naked mole rat's system of "early contact inhibition" still wouldn't apply to human cells. But according to John Sedivy, a molecular biologist at Brown University whose editorial accompanied the naked mole rat paper, figuring out how extreme cancer resistance works in another animal might help us to devise some useful tweaks to our own cellular defenses. Instead of humanizing a mouse to find a better cure for cancer, then, we might try to prevent cancer by mole-ifying a person.
It's early work—and controversial—but Seluanov and Gorbunova won a prize from the journal that published the study: The Proceedings of the National Academy of Sciences named theirs the most important biomedical research paper of 2009. Even with this small triumph, Seluanov says he struggles to find support for his work. The committees that review grants for the National Institutes of Health tend to be "narrow-minded"; they don't like experiments on exotic species such as the naked mole rat and the gray squirrel. "The mouse is an excellent model, ideal for drug development. They gave us these powerful anticancer treatments—chemotherapy and gene therapy. But in order to prevent cancer, you need to look at organisms that are really cancer-resistant," he says. You need to do unconventional research using unconventional tools. "But for them, that's a big red flag."
Buffenstein has faced similar obstacles: "Even to this day, working in naked mole rats, it's quite difficult to get funding. As people at the [National Institute on Aging] keep saying to me, 'It's not that people in program management don't think it's a really exciting project, but the reviewers don't like the fact that you're critiquing the studies that they've done in mice.' "
The lack of dependable support from NIH makes life twice as hard for researchers like Seluanov and Buffenstein, whose work is already slower and more expensive than that of their peers. A cage full of naked mole rats costs four times as much to maintain as a cage full of mice, with fewer reagents and laboratory equipment available for purchase off the shelf or through supply catalogs. Nor can naked mole rat DNA be modified to the extent that's possible—or commonplace—for those using inbred mice. "I'm very envious of the fact that mouse models have transgenics," says Buffenstein.
The day before I arrived in San Antonio, scientists in Liverpool, England, announced that the genome of the naked mole rat had almost been worked out, one of the many special-interest organisms whose DNA is now being mapped with high-speed methods. (A more thorough accounting of the naked mole rat genome was published in Nature in mid-October.) This should help researchers find specific genes that are involved in extending lifespans or short-circuiting the growth of tumors. But there's little hope of generating "knockout" mole rats anytime soon. To make a transgenic Heterocephalus glaber, or even a knockout porcupine, scientists would have to know enough about those animals' reproductive biology to harvest their eggs, transform their stem cells, and then merge the two in a dish (before incubating and implanting the embryos). Even if all this could be accomplished—and keep in mind that it's been done in only a handful of species—the production of such animals would be painfully sluggish. To generate a reliable, transgenic line, researchers must breed through at least 10 generations. An inbred lab mouse can start reproducing 8 weeks after it's born; a naked mole rat or porcupine, on the other hand, doesn't reach sexual maturity until it's at least a year old.
That's why the government shies away from nonstandard research to begin with. Public money goes toward the kind of work that is most likely to deliver tangible results; it's invested at maximum return. A brand-new professor with her first R01 grant might buy a few cages of cancer-prone mice and start pressing out data, but someone who wants to study something unusual—motor control in the octopus, say, or the mating habits of a unisexual whiptail lizard—needs plenty of time to work out the basics, to set up her animal in the lab and describe what she sees. Buffenstein's list of publications stretches across many pages, including some of the top journals in the field, but her work on naked mole rats—20 years' worth, at this point—remains in its infancy, focused, to some extent, on first principles. "There's enough work to do in five lifetimes," she says.
The very thing that makes naked mole rats so interesting to Buffenstein—an astonishing vitality that lasts for decades—only makes her research more difficult. "You're caught between a rock and a hard place, because they live so long that your grandchildren have to finish the studies you start." Still, slow science may have rich rewards, and the decisions we make today—on whether to invest in new model organisms or build out the ones we already have—are sure to have profound effects on the (human) generations to come. Last year's announcement of a plan to create and phenotype every possible single-gene knockout of the Black-6 mouse—to build a $900 million community resource focused on the standard animal—may offer exciting opportunities for all those within the ever-expanding "mouse community." But with every dollar spent on digging deeper, with each new grant awarded for this continuing geology, the institutional inertia gets stronger.
The naked mole rat isn't the only promising yet untapped source of knowledge in biomedicine, nor are cancer patients the only ones who might be better served by a diverse approach to science. A few weeks ago, for example, a team of researchers in Boulder, Colo., announced it had found a set of natural chemicals in the Burmese python that might one day lead to new treatments for cardiac disease. A python uses these fatty acids to make its heart swell by 40 percent while digesting a meal. Since human hearts can also become enlarged, at times with disastrous consequences, snake biology might have something to teach us about the leading cause of death in the U.S.
For all their promise, neither the Burmese python nor the naked mole rat can exist on its own, as a stand-in for every other animal in the lab. When Buffenstein tries to give her mole rats cancer, she uses Black-6 mice as an experimental control. When the team in Boulder finally isolated its snake oils, it tested them in Black-6 mice. Even the pythons themselves were treated to the standard strains: Every other week, they got a single Sprague Dawley rat for dinner. To suggest that we don't need mice or rats for research would be as narrow-minded and absurd as saying that mice and rats are all we need.
Yet we seem to be headed further down the mouse path, with fewer chances to explore the side roads of discovery. The python researchers were denied NIH funding for their study, on grounds that it lacked relevance to human health. As Congress debates spending cuts for NIH in the face of a lingering recession, the urge to shrug off exotic animals will only get harder to resist. Politicians can laugh about studies of grizzly bears or fruit flies, but no one ever mocks the drab little mouse. The classic inbred strains, mobilized for the war on cancer many decades ago, may offer the certainty of data and the most efficient means of its production. But one thing is clear: To do battle against the killers of men, they can't march on alone.
*Correction Nov. 22, 2011: This article originally described Bar Harbor as northern Maine.
More from this series: How mouse research could be limiting our knowledge of human disease, and the one mouse who rules over all other mice. Also, a history of rodent mazes. Read the text-only version of all three parts here.










