This post originally appeared on Business Insider.
Using lasers to regenerate and grow body parts sounds like science fiction, but researchers have just demonstrated that it might be a tranformative tool in medicine—or at least dentistry—in the future.
A Harvard-led team just successfully used low-powered lasers to activate stem cells and stimulate the growth of teeth in rats and human dental tissue in a lab. The results were published today in the journal Science Translational Medicine.
Stem cells exist throughout the body, and they fascinate scientists because they have the ability to become different types of cells — which means they have the potential to repair or replace damaged or worn out tissue. Figuring out new ways to make them useful has long been a goal of medical researchers.
Using lasers to make stem cells do their work is particularly appealing, since it’s a minimally invasive technique, only requiring light once the damaged area is exposed. Scientists have theorized in the past that this was possible, since lasers have been shown to stimulate growth for unknown reasons, but this is the first time that the process has been demonstrated and observed.
The ability to naturally regrow dental tissue could transform dentistry, making it possible to regrow teeth instead of replacing them with a substitute like porcelain. But even more amazingly, once it’s better understood, this same technique could potentially be used to heal wounds and regenerate bone, skin, and muscle.
The research is in its earliest stages and has not yet been tested on humans, so it’s far too soon to say whether these futuristic techniques will ever make it to your local hospital. The treatment possibilities raised by these experiments, however, are exciting to contemplate.
Arany et. al.
How it worked
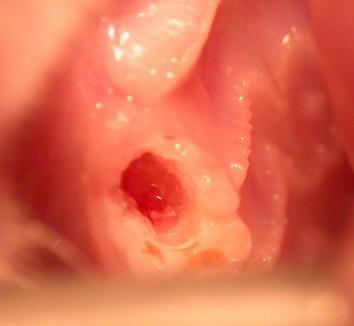
This is the exposed rat molar that received the laser treatment, causing it to start to grow back.
Since the 1960s, doctors have noticed that medical lasers could occasionally stimulate the growth of skin, hair, and other cells. But this is the first time, the researchers write, that the process has been analyzed and understood on a molecular level.
The first step for researchers was to drill holes in two rat molars, exposing the interior of the tooth underneath.
They exposed dentin, which is the harder-than-bone but softer-than-enamel tissue that teeth are mostly made of. Then, they lit up the dentin using a low-powered laser, trying to get the stem cells there to kick into action and start producing more dentin to replace the damaged area.
One molar received the laser treatment, the other did not.
Twelve weeks later, the researchers observed that the dentin in the molar that received treatment was growing again — the tooth was growing back. (The results were the same when they did the experiment again in mice.)
The researchers then tested the same technique on various mammal cells under a microscope. Each time, the laser light caused certain oxygen-containing molecules to appear. Those molecules then caused stem cells to begin their conversion into cells producing dentin, tooth tissue. This showed researchers how lasers can cause tissue to regenerate, which they say had never been seen before.
When tested with human dental stem cells, the effects were similar. The lasers activated the stem cells, which can become many different types of cells, and specifically caused them to start forming dentin.
These three experiments were all focused on the same goal, which was to see if laser light would cause the stem cells in tissue (which had been inactive) to begin the process of generating new tissue. The rat molars showed that it was possible in a living example, while the mammal cells showed exactly how the lasers were working. Using human cells was a way of showing that what was done with rats has the potential to work the same way with human teeth, which the researchers say is the next step in their process.
Why it’s not ready for prime time yet

James Weaver, Harvard Wyss Institute
This model shows how much smaller a rat tooth is than a normal human tooth, though this “rat tooth” is actually a resized image of a human one.
One particular technical challenge of this experiment, according to researchers, was performing oral surgery on tiny rodent teeth. “This is one of those rare cases where it would be easier to do this work on a human,” said David Mooney, a professor of bioengineering at Harvard, in the press release.
New rat teeth didn’t develop perfectly. There was some extra and unnecessary buildup of tooth tissue, which frequently occurs naturally but can potentially lead to painful conditions that need medical attention, including a root canal. However, researchers expect that in humans it would be possible to better cover up areas not being treated, since human teeth are larger. They say that they could more accurately activate certain growth areas without causing widespread tissue formation, but the process has not yet been tested.
The researchers say that they are currently developing human trials for this technique. If those are successful, this could lead to testing laser treatments that regenerate bone, muscle, and other cells throughout the body.
“We are also excited about expanding these observations to other regenerative applications with other types of stem cells,” said Praveen Arany, the study’s lead author and an assistant clinical investigator at the National Institutes of Health, in the news release.
See Also: The Cure for Paralysis