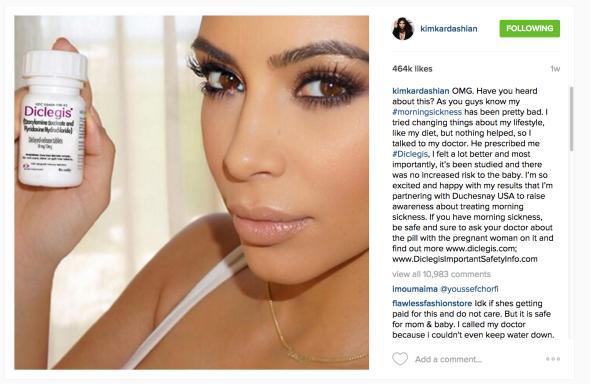
You would think an Instagram post of Kim Kardashian demurely brandishing a bottle of morning sickness pills wouldn’t qualify as one of the pregnant reality star’s more controversial images. But the FDA disagrees: Last week, the regulatory body sent a warning letter demanding that Kardashian take down the offending post immediately. Kardashian had written to her 42 million Instagram followers that her doctor “prescribed me #Diclegis, and I felt a lot better and most importantly, it’s been studied and there was no increased risk to the baby. I’m so excited and happy with my results that I’m partnering with Duchesnay USA to raise awareness about treating morning sickness.”
So what made the FDA so queasy? It seems that Kardashian, a paid promoter of the drug, had touted Diclegis’ benefits while failing to mention any of its risks. Her post, which garnered 460,000 likes, “misleadingly fails to provide material information about the consequences that may result from the use of the drug and suggests that it is safer than has been demonstrated,” according to the letter. Those consequences include drowsiness, difficulty operating heavy machinery, the potential for somnolence, falls, and accidents when taken in conjunction with other antihistamines or depressants like alcohol. Kardashian also failed to mention a significant limitation to the once-controversial drug’s use: It has not been studied in women with the most severe form of morning sickness, also known as hyperemesis gravidarum.
“Failure to correct the violations discussed above may result in FDA regulatory action, including seizure or injunction, without further notice,” the letter reads.
Instagram/People magazine
Social media platforms have taken varied approaches to the question of how their users can promote prescription drugs. Twitter’s ads policy prohibits “misleading or deceptive claims” and says that “[a]ll advertisers should be honest and transparent about the product or service they offer. Include ample information and relevant disclaimers about the products you provide.” Facebook claims that it forbids advertising prescription medications altogether. “Ads must not promote the sale or use of the following: Illegal, prescription, or recreational drugs,” its ads policy page reads. In addition, it prohibits “[d]eceptive, false, or misleading content, including deceptive claims, offers, or business practices.”
As for Instagram (which is owned by Facebook): Its policies on regulated goods and services—including prescription drugs—are rather vague. Basically, it instructs users to follow the laws of their land. “If your photos or videos are promoting the sale of regulated goods or services, including firearms, alcohol, tobacco, prescription drugs, and adult products,” it reads, “please ensure that you know and are following the law that applies to you.”
In the Kardashian case, that meant deferring to the FDA, which has struggled with how to regulate advertising on burgeoning social media platforms. In June 2014, it drafted guidelines for advertising prescription drugs and medical devices on social media platforms that include character-space limitations. Those guidelines said that companies must strive to balance truthful and non-misleading claims about their products’ benefits with an equally prominent disclaimer of potential risks. If there isn’t room within the character limit for a “balanced presentation” of both, the company should reconsider using that particular platform.
Acknowledging the brevity of such platforms, the guidelines add that companies may use a concise statement of risk if it is supplemented by a hyperlink to a complete discussion of safety information. While the Kardashian post did include a link to the manufacturer’s risks page, it nevertheless “entirely omits all risk information,” according to the letter. Duchesnay took the warning seriously, removing the post. “Duchesnay USA … acknowledges that its communications, including in social media as in this particular instance, need to be in accordance with applicable rules and regulations,” it wrote in a statement.
According to the FDA guidelines, that would mean writing a few sentences on Diclegis’ risks (not too difficult, given that Kardashian’s original post was 538 characters long, and Instagram reportedly allows photo captions to go as long as 2,200 characters). However, the message might not have been quite as effective if Kim had written something like this instead:
OMG. Have you heard about this? As you guys know my #morningsickness has been pretty bad. I tried changing things about my lifestyle, like my diet, but nothing helped, so I talked to my doctor. He prescribed me #Diclegis, and I felt a lot better. Of course, now I have to make sure I don’t do anything requiring mental alertness like driving heavy machinery because it makes me drowsy. I also shouldn’t breastfeed, if I’m still taking it after the baby’s born, because #Diclegis can cause “excitement, irritability and sedation” in infants who are exposed to it in breast milk. Oh, and I probably shouldn’t be drinking wine or taking other depressants while I’m on it, because, well, I could fall and die.
Anyways, yeah, this drug is a lifesaver you should totally try it too xoxo!
