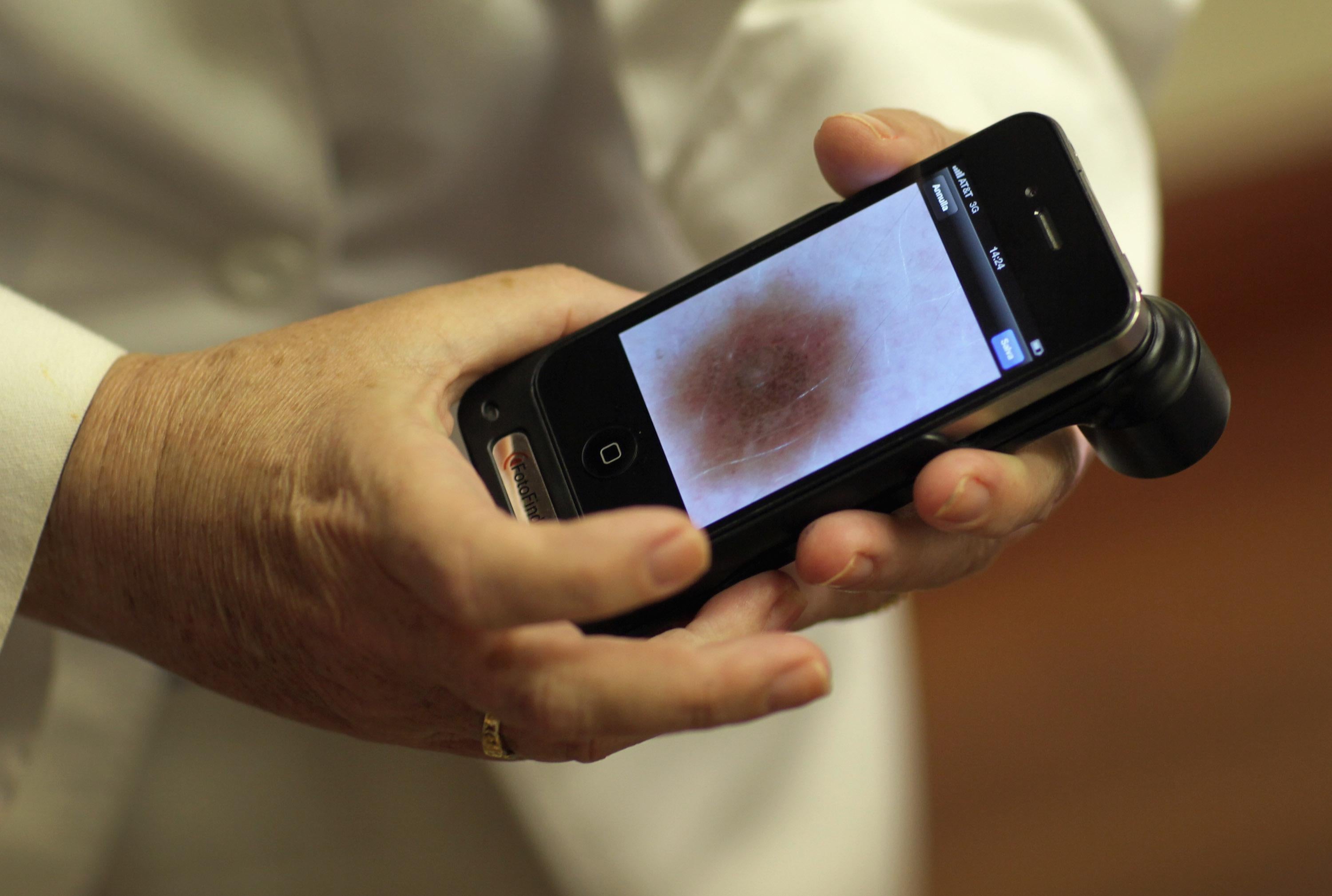
When Apple released the first iPhone in 2007, almost no one saw it as the seed of a medical revolution. But it may have been. Just a few years later, we now see phones that double as cardiac event recorders, blood glucose meters, and remote controls for blood pressure cuffs. Doctors can monitor labor and delivery remotely via smartphone. Quantified Selfers can track their bodies via digestible smart pills or stamplike skin sensors, or their brains via neuro headsets. Worried parents might soon consult mobile devices in the middle of the night or dress their infants in a smart onesie to monitor their breathing and sleep patterns.
If this sounds like science fiction, it’s not by accident. Mobile health, or “mHealth,” draws inspiration from the medical Tricorder—the mythical, futuristic, all-in-one diagnostic device used by Dr. McCoy on Star Trek. One company claims to have created the first real life Tricorder with a puck-shaped device that measures vital signs when held against the forehead. If true, that company could win a $10 million X Prize for the first device that “allow[s] a user to diagnose themselves without having to visit a doctor or hospital.” The winner must also feature a human check engine light to alert users when something is wrong. That might jostle someone wearing Google Glass (which itself has health care ambitions).
But mobile health is not only the fixation of futurists, gadgeteers, and financeers. It also intrigues leading medical thinkers and policymakers, many of whom believe that mobile health technologies can save millions of lives, billions in spending, and democratize access to health care. Almost a dozen federal agencies are interested in it. Perhaps its biggest fan was former FCC chair Julius Genachowski, whose public statements on mobile health read like ad copy. The common refrain among policymakers is: We’re here to help.
But to reach its immense potential, mobile health will need the federal government to act like regulators, not just cheerleaders.
The obvious choice for that role is the U.S. Food and Drug Administration, which is charged with ensuring that the nation’s medical devices are safe and effective. On Monday, the FDA took a step toward that charge when it finalized a much-anticipated guidance on how it will regulate medical apps, many of which qualify as “devices” under FDA jurisdiction.
The FDA’s guidance is sensible enough—the agency says it will regulate only the narrowest subset of apps on the market, mainly those that pose a risk to patients if they don’t work as intended. For example, an app that miscalculates an insulin dose might cause dangerously high or low blood glucose levels in diabetic patients. The FDA’s guidance goes out of its way to clarify which apps the agency will and will not regulate, perhaps responding to criticisms that its first draft in 2011 was too opaque.
But such guidance is necessary. The law that gives FDA authority to regulate devices was written in 1976, when no one outside of Star Trek writers contemplated the mobile devices used today.
Finalizing the guidance was no small feat. After FDA published the first draft in 2011, Sens. Michael Bennett, D-Colo., and Orrin Hatch, R-Utah, tried to prohibit the FDA from finalizing its guidance for 18 months. The aim was to “slow things down” on federal regulation. And earlier this year, the House Energy and Commerce Committee held three days of hearings on wireless innovation in health care, which further warned the FDA not to stifle innovation. The FDA has been pressured by Congress and the industry to tread very lightly.
This reflects the present myopia with mobile health. One estimate pegs the current number of health apps at 97,000. The number of apps and users and downloads seems to double each year—as do sales. By 2015, some predict that 500 million people worldwide will use health apps. Not all health apps fall under FDA jurisdiction. But arguably the most important ones do.
How can the FDA, then, help the mobile health industry from churning out digital snake oil, given the mounting evidence that many apps don’t work? Now that the FDA guidance is finalized, attention will shift to how regulators police the industry.
To date, FDA has cleared about 100 medical apps for marketing. But it has taken very few enforcement actions. The first was in May 2013, when FDA notified a company that its automated urine analyzer app had not received FDA clearance. Before that, the FTC brought its first cases, targeting apps that claimed to treat acne via flashing lights from the phone’s screen. But t
hese three enforcement actions are drops in the bucket considering the number of apps available.
Congress is full of bluster warning the FDA and other would-be regulators not to stifle innovation in mobile health. But if Congress really wants to catalyze a real life Tricorder, it should empower the FDA, not hamstring it.
To start, the FDA barely has the resources or personnel to regulate traditional products in its jurisdiction, not to mention these new ones. Congress should give the FDA the resources it needs to police the massive and growing phalanx of medical apps. Though this may be a short-term inconvenience—particularly to apps that are ineffective or unsafe—it will preserve consumer confidence and encourage high-quality innovation in the long run.
Future Tense is a partnership of Arizona State University, the New America Foundation, and Slate.