Electricity is as blunt a tool as we have in our medical arsenal. Whether it’s an implanted pacemaker or defibrillator paddles on the chest—CLEAR!—using electricity to kick-start a heart feels like getting kicked by a Clydesdale. We use it because it works, but we can’t stop all those volts from ripping through surrounding flesh and bone. Scientists call this a global electrical response, and it does a number on the human body, from killing cells to disrupting the heart’s normal rat-a-tat rhythm. And that’s why a team at Johns Hopkins University is experimenting with a lighter approach.
Literally. Through an emerging field called optogenetics, they plan to use a fancy blue light to coax the heart out of its funk.
“Wouldn’t it be amazing to shine a light on somebody who’s having a heart attack and you’re able to restore their life?” muses Natalia Trayanova, the Murray B. Sachs professor of biomedical engineering at Johns Hopkins University. “We’re far from that, but just the idea of that is really driving this research.”
It sounds fantastical—more like something out of Men in Black than modern medicine—but the study of optogenetics has already made great strides in neuroscience. Now, Trayanova and her team aim to expand the field to cardiology.
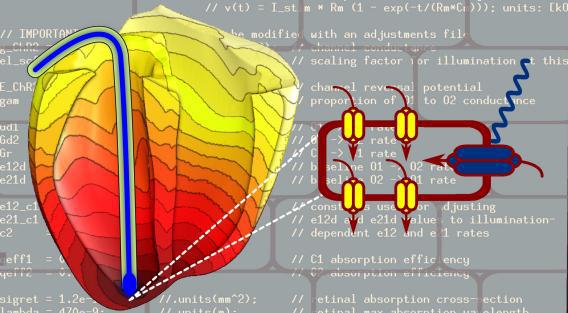
Here’s how it works. The scientists start by inserting light-responsive proteins, called opsins, into individual cells. When exposed to the proper intensity of blue light, the opsins react by opening gateways to the heart’s natural electrical impulses, thus jump-starting the rhythm. A defibrillator works in the same way, though instead of unlatching the gate politely, it charges through like Raiden on a combo.
Treating the heart with this method would also allow medical professionals to target specific areas in need of a low-energy jolt, instead of bathing the whole body in electrical bedlam.
Trayanova and her colleagues are currently testing opsins on an extremely sophisticated computer model of the heart in order to determine the most productive avenues to explore with live flesh. Not only does the model let them see how opsins might perform from the individual cell level on up to the heart as a whole, but in the future it could allow doctors to tailor optogenetic solutions for individual patients.
Since normal human heart cells don’t have opsins, optogenetics won’t be applicable for every patient who comes into an emergency room clutching his or her chest—and we’re likely still a decade away from having this as a treatment option. But for people with increased risk of heart disease or a history of heart attack, this technology has the potential to save lives and improve their quality. “It should be completely painless,” Trayanova told me.
While there’s clearly a lot of work left to do, one thing is certain: As optogenetic heart treatments come closer to reality, you’re going to see a whole slew of headlines talking about lightsabers.