Alan Friedman is a photographer whose pictures of the Sun frequently grace this blog. (See related posts at the bottom of this article.) They are always unusual and beautiful, but even so, this one threw me for a moment:

Photo by Alan Friedman, used by permission
Ewwwww. The Sun is moldy!
Alan took this shot on May 15, and it’s actually a combination of two separate pictures of the Sun using two different filters that show two different physical process on our star.
The outside edge was taken using a filter that lets through the light from warm hydrogen gas. This filter, called an H-alpha filter, accentuates the flow of hydrogen gas under the Sun’s strong magnetic field. Plumes of such material erupt from the surface and when seen against the dark of space are called prominences.
For the solar disk, though, he got tricky. He used a filter that lets through light preferentially emitted by calcium atoms in the solar atmosphere. Calcium under the influence of the Sun’s magnetic fields tends to emit this particular flavor of light, so it traces features called plages, where the Sun’s magnetic field is burbling and bubbling around. Plages look like thin webbing or filaments stretching across the solar surface. In sunspots, the magnetic field can be so strong it shuts off this mechanism, so there isn’t as much light emitted by calcium.
So why do the sunspots look bright and the plages dark? Because for just the disk, Alan inverted the image, making it negative! So in the light of calcium, the dark spots look bright and the bright plages look dark. It makes the Sun look like an orange getting eaten up by mold.
If you invert Alan’s image you see more clearly what I was describing above:
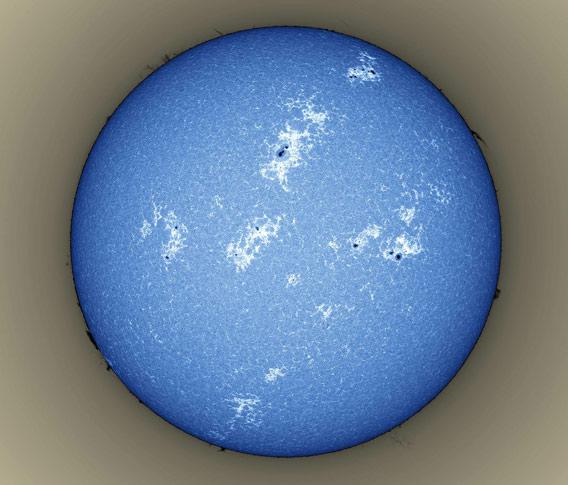
Photo by Alan Friedman, used by permission.
Of course, now the hydrogen light from the Sun’s edge is negative, so the prominences look dark. You can’t have everything. But the light emitted by calcium is at about 400 nanometers wavelength, which looks blue to our eye, so this is closer to what it really looks like.
I’m not sure if Alan’s method has scientific use or not; astronomers sometimes use negative images to see fainter objects, since our eyes are better at picking out dark things against a bright background than vice versa. In this case, though, maybe the artistic value is enough.
So here’s an interesting question: Calcium is relatively rare in the solar atmosphere compared to hydrogen. In fact, there are roughly 500,000 hydrogen atoms for every one calcium atom on the Sun. So why is calcium seen so strongly on the Sun? You’d think hydrogen would be a half-million times stronger!
It has to do with the temperature and density of the solar surface. Hydrogen has a single electron around its nucleus. When that electron absorbs energy in the form of light, it jumps to a higher level, and it can only absorb very specific amounts of energy. It’s like climbing a flight of stairs: It takes a certain amount of energy to get you from one step to the next, and if you lack the energy, you can’t go up. (Try going up a half a step and see what happens—nothing.) The same is true in reverse: The electron emits light at very specific energies when it jumps down from one level to the next.
Calcium is similar, but the energy levels are different; some of the steps the electron can make are smaller and take less energy than for hydrogen. The light we see from the Sun depends on these steps the electrons make, and it’s just easier for calcium to emit (or absorb) that light than hydrogen given the conditions near the solar surface. So even though there’s much less of it, it’s way easier for calcium to get itself noticed.
Also, this particular light is emitted by calcium that has lost an electron in its outer shell (each atom normally has 20 electrons, with two in the outer shell). That means it’s ionized and can be heavily influenced by magnetic fields. That’s why it is a good tracer of the plages, where the Sun’s magnetism is the right strength to yank around the ionized calcium atoms.
Calcium is pretty useful in astronomy. It can be used to trace solar magnetic activity, as well such activity in other stars. This particular light from calcium only comes from stars roughly the same temperature as the Sun, so it can be used to gauge stellar temperatures, too.
And one final note. Guess where all the calcium in the Universe comes from? Exploding stars.
What goes around, comes around.
Related Posts:
Seriously Jaw-Dropping Picture of the Sun
The Boiling, Erupting Sun
No Words
Awesomely Blemished Inverted Solar Beauty
Close-Up of a Solar Monster