Why are we here?
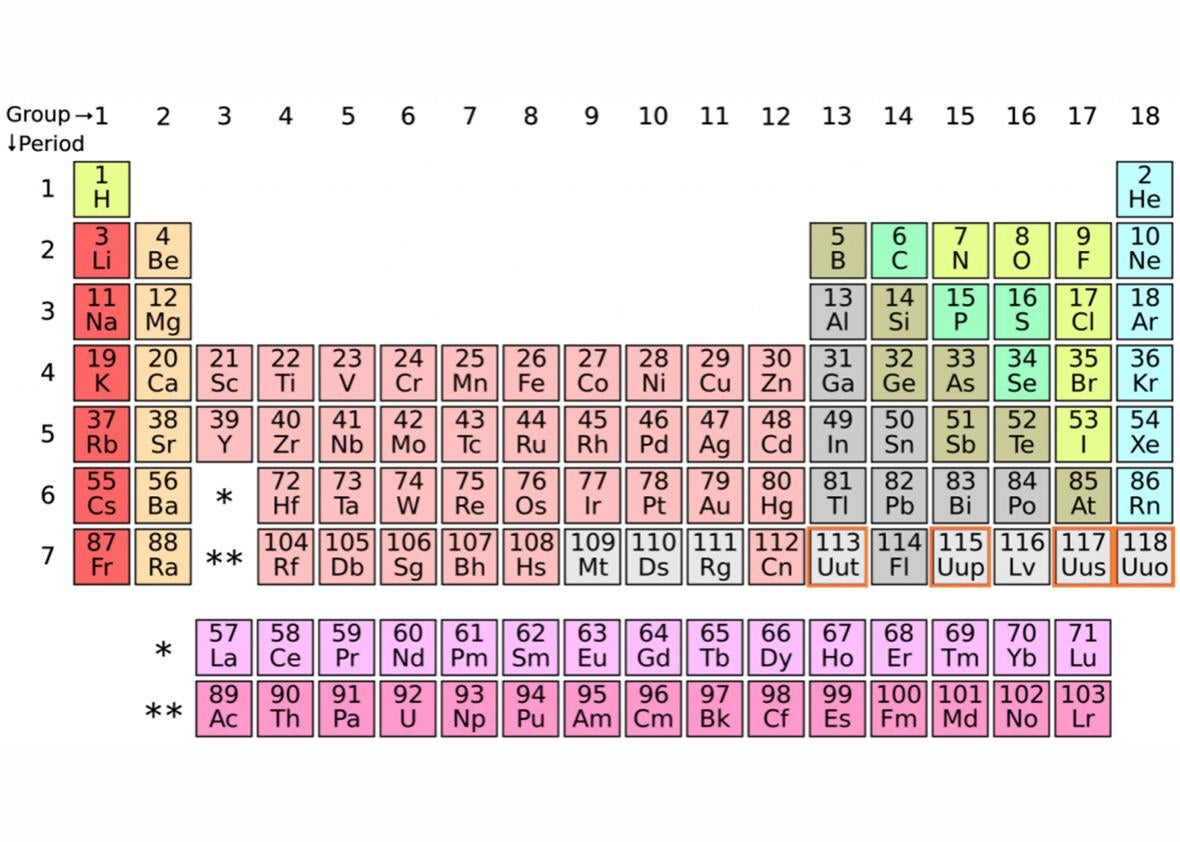
Four new elements are about to be added to the periodic table: nihonium (Nh, element 113), moscovium (Mc, element 115), tennessine (Ts, element 117), and oganesson (Og, element 118).
When you say “new” …
New to the table. Most of them were first synthesized back in 2002 or 2003 (and all of them were theorized to exist long before that).
Wait, I thought elements were the basic core of all of stuff. What do you mean “synthesized”? Or “theorized to exist”?
Let’s back up a bit with a middle-school-science-class refresher: The most basic unit of matter is an atom, with its three main parts: the positively charged proton, the noncharged neutron, and the negatively charged electron. The protons and neutrons hang out in the nucleus, and atoms are defined by how many protons are in the nucleus—that’s where the element’s number comes from (called the atomic number).
Of the 118 known elements, 94 have been found naturally on Earth. The other 24 do not occur naturally, and instead have been made in a lab—synthesized—through a process that involves adding protons onto the nuclei until the total proton count is one that’s never been reached before. Scientists think there could be as many as 170 to 210 elements, depending on how many protons they could add to the nuclei.
How does one go about “adding more protons” to a nucleus?
All the elements left to be discovered, as well as all the elements discovered in recent decades, are superheavy elements that are also very radioactive. Making these involves smashing smaller elements together in the hopes that they’ll fuse. But even when they are created, they’re so radioactive that they’re extremely unstable, sometimes lasting only a fraction of a second. Almost as soon as they form, they disappear.
I see … so do the new elements actually matter for anything? What are we going to do with them, just create them and let them disappear?
For these elements, yes. But there are these things called “islands of stabilities.” Despite the general trend of elements getting less stable as they increase in size, there’s an occasional element with a “magic number” of protons and neutrons that makes it far more stable than it should be, relative to its size. Experts believe the next island of stability is likely to occur somewhere between elements 120 and 126, but that’s just an estimate. It could be element 127, or 171. It could have been 118 (it wasn’t).
Once we reach that next island, we’ll have a superstable element, which may last anywhere from minutes to millions of years (compared with the microsecond lifespans of its neighbors). This could have several industrial applications—for example, it could be a stronger generator of nuclear power. Although we now know there’s not much practical use for the newly discovered elements, we can think of them as stepping stones toward an element that could have practical uses.
If these were discovered more than a decade ago, what took so long for them to get added?
Science is all about reproducibility. Once one team of scientists says it’s discovered a new element (or committed any revolutionary act of science, basically), other scientists have to confirm the discovery by repeating the experiment and seeing if they get the same results. You may have heard that there’s something of a crisis going on in the psychological and biomedical fields, where scientists are unable to reproduce the findings of other scientists. This is a huge problem because if other scientists can’t confirm results by reproducing them, people could just go around lying about what their research found. Like, say, this new element they just discovered.
Has that ever happened?
Yes! And very recently, too. In 1999, a team in California claimed to have synthesized two new elements, 116 and 118 (which has now actually been synthesized and is soon to be added to the periodic table as oganesson), but when other researchers couldn’t replicate the experiment, one researcher admitted he had fabricated the data.
So who gets to name new elements?
Whoever discovered them, of course.
So scientists can just name them whatever they want?
Not exactly. The IUPAC—
Who?
The International Union of Pure and Applied Chemistry. They’re the people who control what goes on the periodic table, among other things.
Oh, OK. Go ahead.
Anyway, the IUPAC says that elements have to be named after one of five things: a scientist, a place, a mineral or substance, a descriptor of the element, or a mythological reference. Of the new elements, three are named after places and one is named after a person.
Wait—mythological reference? You have to give me an example.
There are 15 elements named after a mythological character or reference. Thorium and vanadium are named after the Norse god of war Thor and goddess of beauty Vanadis, respectively. From Greek mythology, helium is named after the sun god Helios, iridium after the goddess of the rainbow Iris, and titanium after the Titans.
That’s not to mention all the elements named after planets (either current or former)—mercury, phosphorus (an old word for Venus), uranium, neptunium, plutonium—which each take their names from mythological characters. Same for the two elements named after asteroids: palladium and cerium.
OK, but none of these new elements are named after mythology. So what’s nihonium named after?
The name is based on the Japanese word Nippon, which is one word for Japan itself—where the element was discovered—and means “Land of the Rising Sun.” Formerly known as ununtrium (this name is based on a clunky and disliked naming convention among chemists to name elements that haven’t yet been discovered or verified by their atomic number—un+un+tri+um=one+one+three+um, or 113, its atomic number), Nihonium has a half-life of 20 seconds, making it the longest-lived of the new elements. Other proposed names included japonium, rikenium (after the institute where it was discovered), and nishinanium (after a Japanese physicist).
Moscovium?
Named after Moscow.
The city?
No, technically, it’s named after the Moscow “oblast,” where the lab that discovered moscovium is located. Oblasts are like U.S. states, so there’s actually a Moscow, Moscow. Kind of like New York, New York. Specifically, the lab that discovered moscovium is located in the city of Dubna.
What was the next one?
Tennessine.
Right. Named after Tennessee?
You got it. Researchers at Vanderbilt University and University of Tennessee–Knoxville teamed up to discover this one in 2010, making it the most recently synthesized element. It’s also just the second element to be named after a U.S. state, the first being californium.
And oganesson?
Oganesson, formerly known as element 118, is named after Yuri Oganessian, a Russian nuclear physicist. Both moscovium and oganesson were discovered in the same place, at the Joint Institute for Nuclear Research. That’s the same research facility where many other elements were first synthesized. In fact, the JINR has discovered or helped discover five of the six most recent elements.
So … some dead guy?
Actually, he’s still alive! He was one of the scientists who helped discover the element. If the proposed name is finalized, oganesson will be the second element named after a living person (the first was seaborgium, named after Glenn Seaborg).
So what’s unique about these elements?
To be honest, the most interesting thing about these elements is probably how unstable they are. They are all radioactive and have extremely short half-lives, meaning the time it takes for half of the amount of the element to disappear. For simplicity’s sake, if you start out with four pounds of a radioactive substance and wait one half-life, you’ll have two pounds of the pure substance left.
We mentioned that nihonium has a half-life of about 20 seconds. Moscovium is even shorter lived, with a half-life of 220 milliseconds. Tennessine’s is just 78 milliseconds. If you want to get an idea of how quickly tennessine decays, fire up your stopwatch app and try to start and stop it as fast as you can. You’ll probably get something like 0:00:15, which is 150 milliseconds. In that time, nearly two of tennessine’s half-lives have passed.
Oganesson—
Let me guess: Its half-life is even shorter.
Uh, yes. Kinda took the wind out of my sails there.
Fortunately, there’s a little more to this one anyway. To date, oganesson is the heaviest known element, and it doesn’t react or bond easily with other elements. It’s also on the far right of the periodic table, in a column (or “family”) of noble gases, like neon and argon, which are notoriously inert, rarely reacting with anything.* For very complicated reasons, though, Dubna researchers theorize that oganesson is actually a solid, which would make it the first known “noble solid,” a solid version of a noble gas. It’s hard to be sure though, since only three, maybe four atoms of oganesson have ever been observed.

PeterHermesFurian/iStock
So what happens next?
Well, if you look closely, you’ll see we filled up the last row of the periodic table. That means when the next element is discovered, we’ll have to redesign the table … slightly.
What a headache. How soon is all of this over?
The names were announced on June 8, and the public has five months—until Nov. 8—to voice any concerns about the proposed names. After that, IUPAC is expected to formalize the names and update the periodic table.
But these names are final, right? The public only gets to object, not vote?
After the disaster that was Boaty McBoatface, that seems to be the prudent approach, yes.
*Correction, Oct. 16, 2017: This piece originally misidentified a column on the periodic table as representing a “period” when in fact it represents a “family.” (Return.)