This post originally appeared on Wired.
It’s not the onion’s fault you’re crying. The wasabi isn’t to blame for jolting your sinuses. And don’t curse the hipsters outside the bar for the burning cough you got walking through their cloud of cigarette smoke. Those things are actually all your fault. Or rather, those uncomfortable sensations all trace back to special proteins on neurons inside your body. Those wee tangles are why you cry, cough, sting, itch, swell up, or burn whenever you encounter something noxious.
And it’s for your own good, you know. These little proteins—called TRPA1 receptors—just want to keep your body from harm. Those various responses are supposed to make you feel uncomfortable, so you’ll spit out, cough up, or get the hell away from the danger. TRPA1 is a major part of the body’s pain system. And Wednesday scientists announced a breakthrough: With the help of major advances in electron microscopy, they were able to build a complete 3-D model of TRPA1’s structure. Every spiraling alpha helix, every knobby amino acid, all the nooks and crannies. This isn’t just a score for basic biology; it could lead to drugs that are better at targeting pain while causing fewer side effects.
In a sunny room on the third floor of a research compound at the University of California–San Francisco’s Mission Bay campus, David Julius—the molecular biologist whose passion and expertise led to this discovery—is drawing on a white board. First a big circle, and inside it a little circle: a cell. He adds a small rectangle to the larger circle and labels it TRPA1. “From the top down the structure looks like a donut,” he says. Really, it’s more like a sphincter. Because normally TRPA1 is clenched up tight. Only with the right chemical cues does it open up, letting ionized sodium and potassium into the cell. “This generates an electrical current, and allows the neuron to send a signal to the central nervous system,” says Julius in his lightly Brooklyn’ed accent.
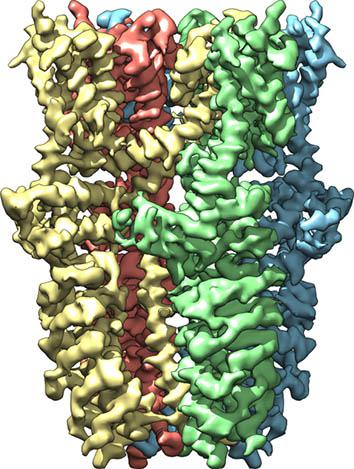
Image by Jean-Paul Armache/UCSF/Wired
Zap! Something bad is in your body, and TRPA1 trips the alarm. Your body’s response depends on equal parts data (there’s something bad in the body!?!) and metadata (where is this bad thing?!?). Which is why TRPA1 is responsible for such a wide range of (mostly negative) sensations. A dollop of wasabi is going to make your mouth and sinuses feel like you’re prodding them with a knitting needle. If you find yourself downwind of a campfire, thank TRPA1 for your watery eyes. Likewise for why your lungs feel like they’re on fire when the cops start firing tear gas into your crowd peacefully protesting against the 1 percent (hypothetically, of course). “The molecular response is the same. Irritant molecules activate TRPA1,” says Sven-Eric Jordt, who studies pain response pathways at Duke University. “But the way the body interprets it is based on the location of these nerve endings.”
TRPA1 is part of a family of receptors collectively known as the transient receptor potential (TRP—get it?) channels. They all operate the same way—chemical stimulus from something bad produces an electrical current—but each has different triggers. And for two decades, Julius, or an alumnus from his lab, has been behind nearly every major research breakthrough involving this receptor family. This includes the discovery of the most famous sibling: TRPV1, the “chili pepper sensor.”
Like TRPV1 (which senses not just chemical heat from peppers, but also actual heat, the thermal kind), TRPA1 is versatile. It picks up cues from not just foods like wasabi, onions, and mustard oil, but also things like burning vegetation, vehicular exhaust, and even inflammatory signals created within the body. It’s also thought to play some kind of role in the itch that comes from poison ivy. Julius says that every animal has receptors analogous to TRPA1, and in each species the proteins have evolved slightly different sensitivities. Pit vipers—a family of snakes including rattlesnakes and moccasins—use infrared-sensitive, TRPA1-laden neurons to sense body heat coming off prey in the dark.
How They Did It
Arguably just as important as how the wasabi receptor works is how Julius and his team figured it out—using a game-changing technology called cryogenic electron microscopy, or cryo-EM. A few floors below the sunny office with the white drawing board, Yifan Cheng—Julius’ compatibly obsessive co-author—points at the top third of a large, cylindrical microscope that almost touches the ceiling. Electrons are fired from up there and interact with a frozen specimen at chest level. It’s the only way delicate TRPA1 proteins can survive the vacuum conditions necessary for electron microscopy.
Next, a special camera, the part of the microscope nearest to the ground, sees what no other camera can. Old electron microscopes convert the electrons they used to look at teeny, tiny things into photons—light—so people could actually see them. But because a single electron would generate multiple light particles, the images were blurry. Cheng’s microscope doesn’t have that problem, because it uses silicon to image the electrons directly. Kneeling, he points at the logo on his device: a mountain silhouette labeled K2. “K2 is the hardest mountain in the world to climb, harder than Everest,” he says. “Making the camera was a very difficult project.”
Cryo-EM is having a major moment, giving scientists their first detailed views of the ubiquitous, critical structures that let chemical messages in and out of cells. For most of the history of molecular biology, electron microscopy played second fiddle to X-ray crystallography (the technology Rosalind Franklin used to view the first DNA structure). But membrane proteins like TRPA1 are too fatty and unruly to arrange in the exacting alignments necessary for crystallography. Electrons, on the other hand, detect the proteins no matter their orientation.
This kind of detail means cryo-EM is going to be useful for much more than basic science. “You can eventually get to see where drugs will bind,” says Julius. Today researchers develop new drugs essentially through trial and error, testing new compounds against targets without really knowing what those targets are. Potentially the kind of detailed view cryo-EM yields can give them a better shot. “Since TRPA1 is also involved in inflammatory pain, this breakthrough may lead to structure-based inhibitors that can treat pain and inflammation,” says Jordt, who was not an author on this paper. (However, he was a post-doc for Julius in 2004. Suffice to say, it is incredibly difficult to find TRPA1 experts with no connection to David Julius.)
But Julius and Cheng consider themselves basic scientists, more interested in understanding how the body works than making new drugs. And TRPA1 still has a lot of secrets, not even counting the ways wasabi, garlic, and car exhaust trigger human reactions. “Like, how is an ion channel activated by heat?” Julius says, thinking of the pit vipers and their unique TRPA1 adaptation. “There are a lot of rattlesnakes out there that need our help.”
Also in Wired:
This Guy Says He Can Make 20-Year-Old Rum in Six Days
Holograms Will Let Doctors See 3-D Views of Our Insides
Scientists Say It’s Time to Reinstate the Brontosaurus
Why Does Stubbing Your Toe Hurt So Damn Much?
The Deadly Global War for Sand
