This year’s Nobel Prize in chemistry has been awarded to three scientists whose work has greatly improved what we can look at under a microscope. Things that were once thought too small for optic magnification—such as the structures of proteins, the movement of viruses—can now be observed by the human eye and captured through the lens of a camera.
Light (or optic) microscopes use a light source and a system of lenses to bring the miniature into view. But they are constrained by natural properties of optics: Image resolution is limited by the wavelength of light used—anything smaller than about 0.2 millionths of a meter (or 200 nanometers) could not appear any clearer than a blob. (A different kind of microscope, called an electron microscope, does not have this size restriction, but it can only look at specially fixed samples, not living cells or organisms.)
Stefan Hell of the Max Planck Institute, Eric Betzig of the Howard Hughes Medical Institute, and William E. Moerner of Stanford found ways to overcome this so-called Abbe limit (named after Ernst Abbe, the German optician who proved it in 1873) by using another tool: molecules that fluoresce. Hell’s technique is called “stimulated-emission depletion.” A tiny structure, such as a protein, is treated so that it glows when zapped with a beam of a particular wavelength. A second beam quenches the glow, except in a small area. These two beams scan the whole specimen this way, allowing a picture to build up with a clear resolution, even for structures fewer than 200 nanometers apart.
Betzig’s method, on the other hand, takes multiple pictures of a sample that has been tagged using special fluorescence properties discovered by Moerner. Different molecules of the sample light up with each picture, and stacking these images on top of one another also builds up a much finer-revolved image than traditionally possible. Two powerful microscopy methods that have since been developed called “photo-activated localization microscopy” and “stochastic optical reconstruction microscopy” use this principle. Images made possible by the work of this year’s chemistry Nobel Laureates are showcased below.
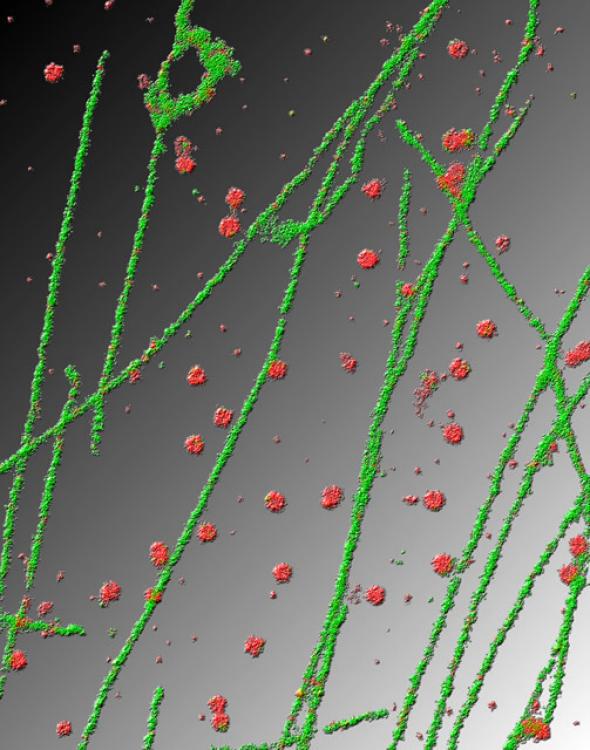
Courtesy of Xiaowei Zhuang/Harvard University/Nikon
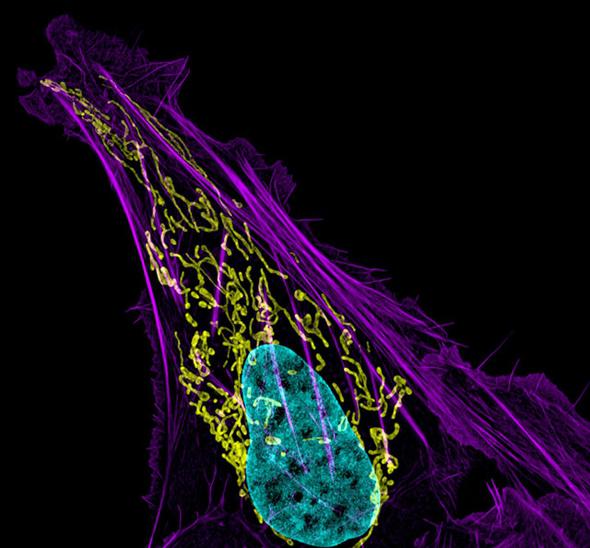
Courtesty of Dylan Burnette/NIH/Nikon
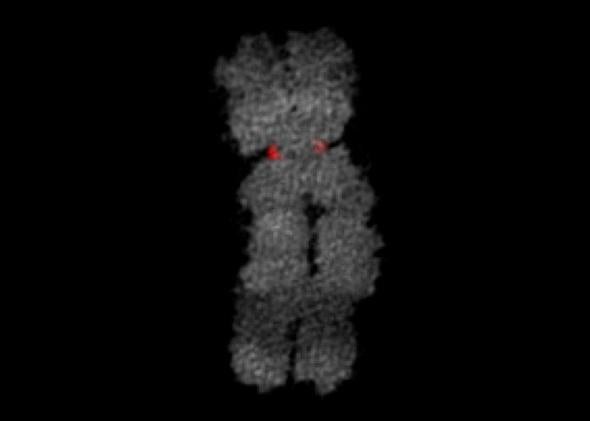
Courtesy of Michael Huebner and Zsolt Lazar/Nikon
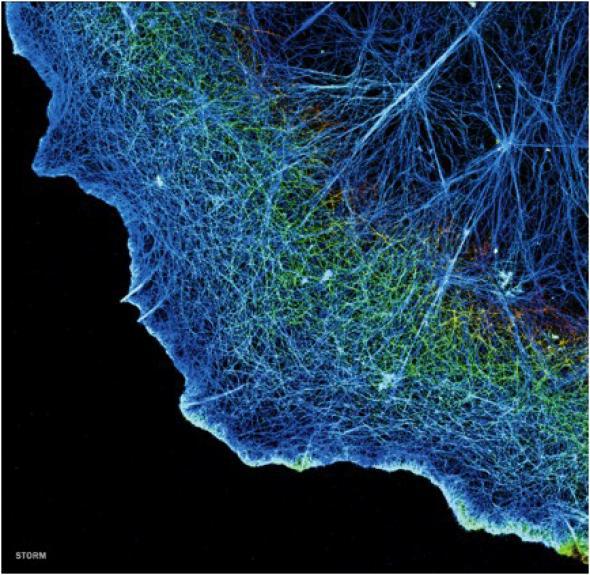
Courtesy of Xiaowei Zhuang/Harvard University/HHMI
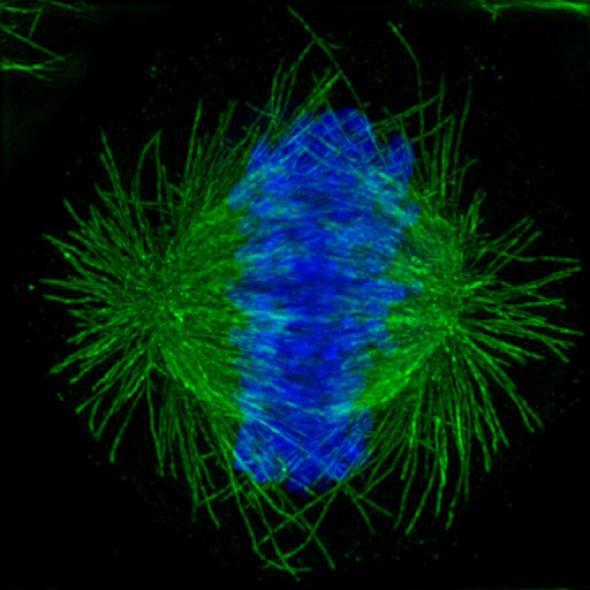
Courtesy of Lunenfeld-Tanenbaum Research Institute at Mt. Sinai Hospital
Correction, Oct. 10, 2014: The caption of the human bone cancer cell image originally mislabeled the colors of the mitochondria and DNA. The mitochondria is yellow, and the DNA is blue.